- Top Page
-
About
About
Clinical
Biostatistics
Course
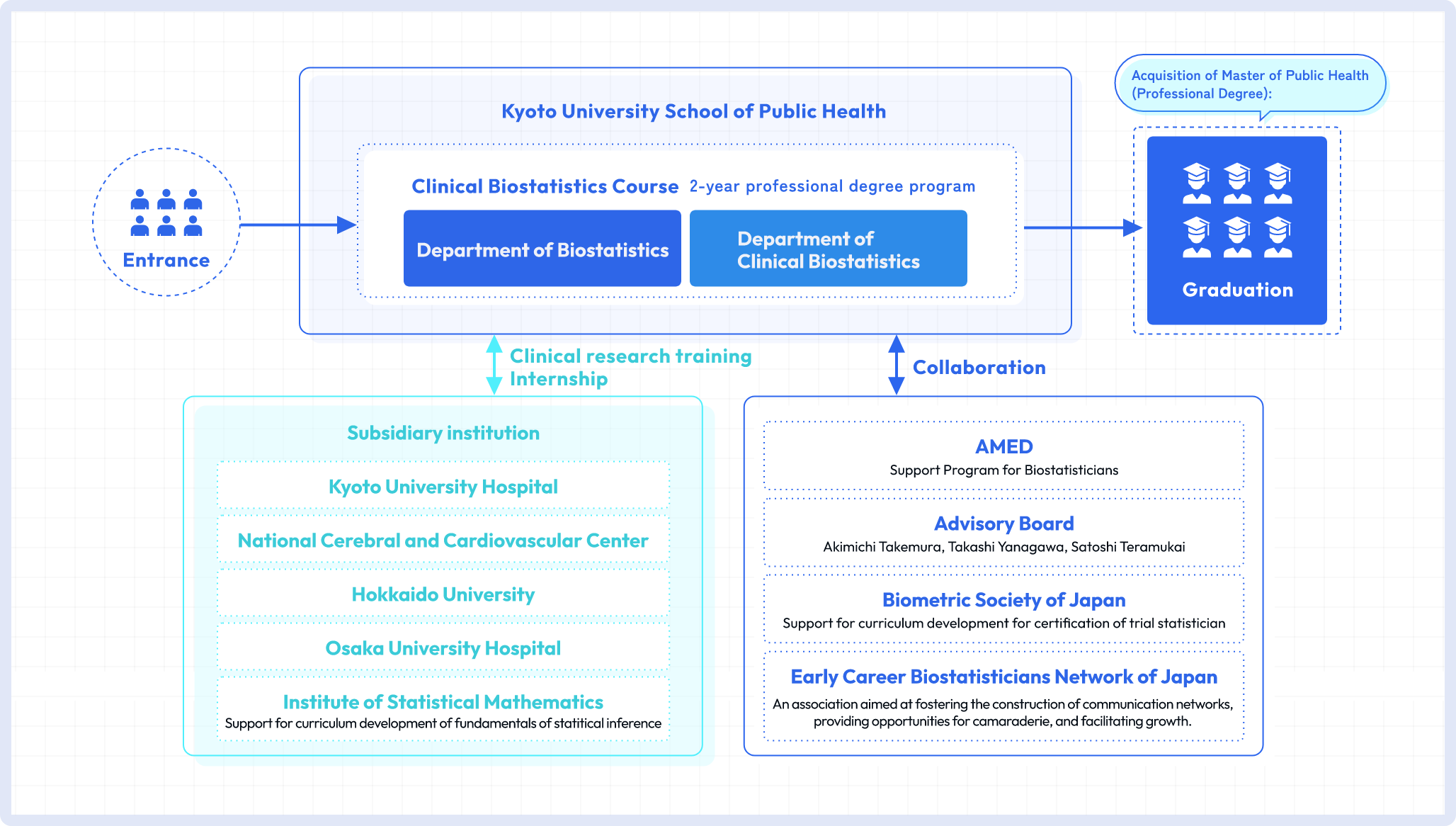
This two-year postgraduate professional degree program is designed to equip students with the knowledge, skills, and attitudes required to work as a biostatistician. It is offered by Kyoto University School of Public Health (SPH) and jointly managed by the faculty members of the Department of Clinical Biostatistics and the Department of Biostatistics. The Institute of Statistical Mathematics contributes to the program design to ensure the quality of education on the foundation of statistics. The Kyoto University Hospital, National Cerebral and Cardiovascular Center, Osaka University Hospital, and Hokkaido University Hospital participate in the on-the-job training modules. Additionally, this course collaborates with research institutes that employ graduates to provide postgraduate education to support their careers as a biostatistician.
Biostatistician








A professional with highly ethical, scientifically objective minds
Clinical biostatistics provides the methodologies used to design clinical trials and analyze their data. High ethical standards and rigorous scientific objectivity are required to work as a biostatistician who participates in clinical trials. Pharmaceutical and other medical products are developed by academia and industry and must be confirmed for efficacy and safety in clinical trials before they can be approved for marketing. In such clinical development, rigorous statistical approaches are required to draw scientifically valid conclusions, and therefore biostatisticians with expertise in methodological and mathematical aspects of clinical trials are indispensable in successful drug development. Biostatisticians’ responsibilities cover practical and theoretical aspects of clinical trials. They participate in designing the study protocol, conducting interim and final statistical analyses (including statistical programming and reporting), and interpreting analytical results. Most of their activities are carried out in collaboration with medical professionals and require general medical knowledge and communication skills. The theoretical work of biostatisticians relates to developing and evaluating new statistical methods through mathematical derivation, computer simulation, and application to real datasets. To support high-quality clinical trials, clinical biostatisticians need practical skills and theoretical knowledge in a wide range of topics including statistics and study methodologies.













